Division of Endourology, Robotics, Laparoscopy and Minimally Invasive Surgery, Department of Urology, University of Miami, USA
Abstract
The surge of technological advancements has changed the way we treat diseases. In the realm of nephron sparing surgery there is a raging debate on the role of ischemia in the preservation of renal function. We set about discussing this issue by conducting a literature search of pertinent articles of interest. Our narrative synthesis of these articles sheds light on the matter as well as discusses recent innovation that modifies or even eliminates the use of mechanical ischemia in renal surgery.
Keywords: Partial nephrectomy; Nephron sparing surgery; Warm ischemia
Introduction
With the advent of cross sectional imaging, we have witnessed a surge in incidental detection of Small Renal Masses (SRM). The rise of early detection of clinical T1a and T1b cases fortunately has coincided with technological advances that have empowered urologists to extirpate tumors with less collateral damage and nephron loss than possible with total nephrectomy. Scientific studies have also suggested that Radical Nephrectomy (RN) may condemn select patient populations to renal insufficiency [1]. Current literature has demonstrated the efficacy of nephron sparing surgery producing similar oncologic outcomes and better functional results [2]. Hence, the recommended modality of treatment for SRM has shifted from, radical nephrectomy to Nephron Sparing Surgery (NSS) [3]. A fundamental issue in the conduct of this treatment modality is the widely accepted role of ischemia in the resultant renal function of the treated kidney. A more contemporary theory is that it is the volume of remaining parenchyma after Partial Nephrectomy (PN) or Thermal Ablation (TA – i.e., Radiofrequency or CryoAblation). The concept overlaps with traditional thinking as there is a correlation between tumor complexity and subsequent collateral damage suggesting tumor characteristics are more important than simply warm ischemia time (WIT). We set about addressing this issue by reviewing recent articles pertaining to the impact of ischemia in the preservation of renal function.
Methods
A literature search on Science Direct was performed with the following key words: “warm ischemia” and “partial nephrectomy”. We purposely excluded studies in which cold ischemia was introduced as a treatment parameter. The search revealed more than 1,400 articles. We selected prospective, retrospective or experimental studies written in English. We also limited our search to articles published from 2004 to the present time. This is to allow for patient accrual from 2002 when the technique of robotic partial nephrectomy was first introduced. The READER critical appraisal tool (Macauley, Queen’s University, Belfast, Northern Ireland) was used to evaluate the articles. After establishing the relevance of these articles we then commenced with our narrative synthesis.
Results
With these parameters outlined in our methods, we selected 15 articles to review. These may be classified to 7 prospective studies, 3 animal experimental studies, 3 retrospective studies and 2 editorial articles. Of the 13 studies, 12 are non-randomized trials and only 1 is a randomized controlled trial.
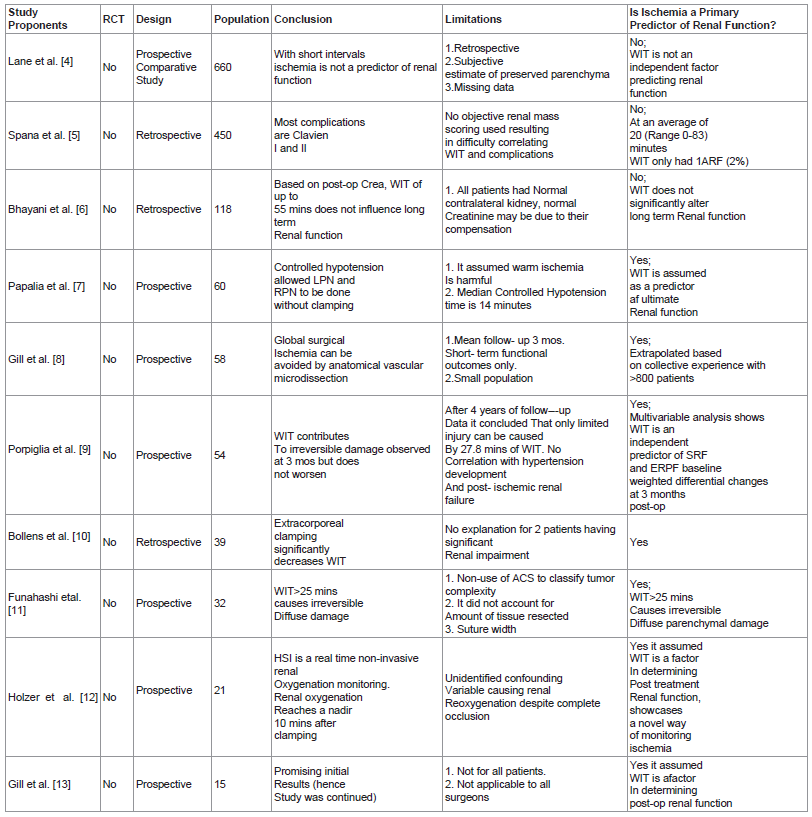
Table 1 lists the 15 articles included in this review and includes a brief description of their research design, conclusions and study limitation.
Discussion
Of the 15 articles the most influential is that of Aron et al., who reported their series of 58 patients in which global surgical ischemia was avoided using an anatomical vascular dissection technique [8]. This study which at the onset has considered warm ischemia time (WIT) as the primary predictor of post treatment renal function is actually a continuation of this group’s first publication on the technique in which they presented their initial 15 patients [13]. The premise of considering warm ischemia as the primary predictor of post treatment renal function was extrapolated from the conclusion of their previous case series of 800 patients that underwent laparoscopic partial nephrectomy [19]. This most recent article was cited by two other publications [12,17] included in this review and is actually the topic of debate of the editorial on opposing views [18]. In this pool of publications this article with a modest population has caused the most controversy. Its main contention of preventing unnecessary global ischemia through anatomical vascular dissection was well explained and its advantage of allowing even more complex tumor to be safely excised without hilar clamping through clever use of intraoperative doppler ultrasonography coupled with a preoperative 3—D video road map reconstructed from 0.5 mm cut CT images was well elucidated. It is a remarkable documentation of a single surgeon’s negotiation and overcoming of the steep learning curve for laparoscopic and robotic partial nephrectomy as plotted by their technical improvement gradually decreasing and eventually eliminating warm ischemia. The paper despite its encouraging short-term functional outcomes has shortcomings as admitted by its authors. A larger population size and a longer follow-up are recommended. The use of MAG-3 to evaluate all post-operative renal function would provide stronger evidence of renal function preservation and a prospective design would be the ideal method of conducting this study. The study proposes transfer of this technique to other robotic surgeons. The authors suggested a gradual advancement towards super selective vascular microdissection.
Table 1: lists the 15 articles included in this review and includes a brief description of their research design, conclusions and study limitation.
Dr. Parekh’s editorial article [17] is a well outlined rebuttal of Aron et al’s paper. Indeed all practicing urologists subscribe to the basic principle that renal ischemia is harmful. The traditional cut off time for ischemia of 30 minutes is being contested by recent studies. One article in this review documents detrimental effects to renal function when ischemia time exceeds 25 minutes [11]. Dr. Parekh claims that as a reaction to these cautionary literature the profession has developed intolerance towards ischemia which are now fueling significant changes in surgical approaches. He urges everyone to pause in this pursuit towards selective clamping of renal vasculature and carefully assess the evidence. He cited Lane et al’s study [4] that reported ischemia duration and type are no longer predictors of renal outcomes but instead it is the quantity and quality of remnant renal parenchyma that are important. He further emphasized that these data were gathered in the context of a solitary kidney where the need to limit the duration of ischemia is widely accepted. Citing another study [20] this time coauthored by Dr.Lane and Dr.Gill, he argued that in the usual scenario of a patient with bilaterally functional kidneys again the quantity and quality of the renal parenchyma rather than an ischemia time of less than 30 minutes predicts renal functional outcomes. He went further by comparing Dr.Gill’s perioperative outcomes to a recently published multi-institutional series of robotic partial nephrectomies [5] to illustrate that increasing procedural complexity does not come without any trade offs.
Dr. Gill’s contribution on the article of opposing views provides a good closing argument for his case. He admits that common sense would dictate that kidney quantity and quality fundamentally drives ultimate function after partial nephrectomy. He contends however that the main issue of this debate is whether it is worthwhile to minimize renal ischemia. He cited Thompson et al. paper [21] which documented that every minute of ischemia increased the risk for acute kidney injury and new onset stage IV chronic kidney disease. He criticized Lane’s paper [4] which reported that ultimate kidney function correlated only with percent parenchyma preserved and preoperative GFR and not ischemia time as flawed since parenchyma preserved was subjectively assigned intraoperatively by 21 surgeons in 4 institutions. This flaw makes the vital parameter being measured a non-standardized variable since the method used acquiring it does not account for inter-observer variability. He further argued by re-citing Thomson’s paper [21] which took into account percent kidney preserved but still demonstrated the persistent effect of warm ischemia time (WIT) although secondary only in magnitude to quantity and quality of parenchyma spared. He claims falling second to quality and quantity of remnant parenchyma does not equate to being irrelevant. Between parenchymal characteristics and ischemia, only ischemia is the surgically modifiable factor. He further elucidated the impact of ischemia on renal function by making a reference to aortic aneurysm reconstructive surgery where renal characteristics are fixed and ischemia is the only new variable. In these cases short term ischemia led to a reduction of renal function by as much as 1/3 of baseline eGFR. He also reemphasized the fact that renal metabolism is primarily aerobic and ischemic along with reperfusion injuries can cause acute kidney injury which worsens underlying chronic kidney diseases. He drives down his point that there is nothing beneficial about renal ischemia and since it is our mandate to preserve the maximum number of nephrons in the best condition possible then eliminating ischemia may even be better than just minimizing it. He urges everyone to raise the surgical bar and pursue zero ischemia partial nephrectomy as the technique of choice in treating small renal tumors.
Dr. Campbell’s side of the opposing view article provides a less visceral, factual, summation of all the arguments against the non-ischemic approach. He refocuses on the main goals of partial nephrectomy which is cancer control and functional preservation done through a process that affords minimal morbidity. The standard partial nephrectomy technique achieves all these with limited ischemic intervals. He admits that the non-ischemic partial nephrectomy does stimulate great interest. He cautions however to examine its potential disadvantages and questions the core driving principle of this technique which identifies ischemia as the main determinant of post treatment renal function. He cites the same article by Thomson et al. [21] which reported the directly proportional relationship between WIT and new onset Stage IV CKD and reveals the major flaw in this study. He explains that the analysis in this literature did not take into account the percentage of preserved parenchyma and in its absence designated WIT as its surrogate in the role of predictor of ultimate renal function. He admits that there is a strong correlation between WIT and quantity of preserved parenchyma. He explains that a simple tumor that has a significant amount of renal parenchyma preserved will have a short WIT. Conversely a complex mass that leaves less parenchyma would have a longer WIT since it would require a more elaborate reconstruction. Statistical evaluation however, affirms preserved parenchyma as the independent predictor of ultimate renal function. He further argues that studies supporting the non-ischemic approach are misleading since these did not take into account volume loss in their analyses and that these literature are flawed since the authors imply a cause and effect correlation while the data can only support a correlation. He argues that a limited WIT can provide good vascular control facilitating a more precise and safe partial nephrectomy. He emphasized that WIT is not irrelevant but limited amount of it is not as harmful as previous literature suggests.
Our analysis of this debate is that “where to clamp” is at the crux of the argument. All urologist’s that deal with renal cancer adhere to the basic tenets of nephron sparing surgery: 1) to save and preserve as many nephrons as possible and 2) that ischemia can be thought of as a tool. It allows a clearer operative field which facilitates oncological outcomes and repair, whilst also preventing blood loss. Both zero ischemia and hilar clamping techniques employ clamping. They just differ in the location. The choice of technique to employ depends entirely on the surgeon’s preference. If one has the skills and equipment to dissect the tertiary and quarternary vascular structures of the kidney then nephron sparing surgery via the zero ischemia technique should be one’s best option. On the other hand, if one is comfortable with the current and usual method of performing partial nephrectomy via hilar clamping, and you can do this within the allowable amount of ischemia time, then performing partial nephrectomy through the conventional method would serve your patient well. As to the issue of surgeon transferability of the zero ischemia technique, we think it is not yet applicable to everyone. There are 3 factors to consider: first is the volume load of the surgeon’s center; second is logistics, one must have access to the necessary imaging devices that can help you execute the procedure; and lastly, your intrinsic surgical capability. Negotiating the learning curve does not only involve repetitive performance of the same procedure over time but also involves the factor of intrinsic surgeon skill or capability to learn and adapt a new technique. As to the contention that adapting the zero ischemia technique is tantamount to raising the surgical bar, we think further studies on this technique is warranted before we brand it as the new gold standard in nephron sparing surgery. As of the present time as Parekh [17] recently pointed out even the main proponent’s statistics of operative parameters and peri-operative complications in patients done through zero ischemia technique does yet equal his own statistics of the same criteria in patients done through the standard multiport laparoscopic or robotic partial nephrectomy. If in the next 100 cases of zero ischemia, we actually see an improvement that would make the proponent’s own statistics equal to his standard LPN or RPN numbers then the possibility of zero ischemia as the next gold standard is in the horizon.
Lane et al. [4] article was a non-randomized, comparative study that consisted of 660 patients who underwent open partial nephrectomy with either cold or warm ischemia in 4 participating institutions. Their results revealed that at 3 months post-op median GFR decreased by 22% in both warm and cold ischemia groups. Multivariable analyses revealed the following factors to be associated with decreased post- op GFR: increasing age, larger tumor, lower baseline GFR and longer ischemia time. But when percentage of the parenchyma spared was included in the analysis, this factor came out to be the primary determinant of ultimate renal function. As Baldwain et al. [15] pointed out the main criticism for this paper is its lack of accounting for interobserver variability for assigning the percentage of parenchyma spared. Despite this shortcoming however, we feel that this paper holds merit in this issue of the role of ischemia in nephron sparing surgery.
Spana et al. [5] presents the intraoperative and postoperative complications of 450 robotic assisted partial nephrectomy in 4 participating institutions. At a mean WIT of 20.2 minutes this study revealed a 15.8% complication rate. 76.1% of these are Clavein I-II while only 23.9% are grade’s III-IV. Since most complications can be managed conservatively, this study concludes that robotic partial nephrectomy is a safe and reliable technique. It is also interesting to note that in this series with a WIT range of 0-83 minutes, only 1 patient or 0.2% of the population developed acute renal failure which did not require dialysis due to its transient nature.
Bhayani et al. [6] reports 118 patients who underwent laparoscopic partial nephrectomy and subgrouped them into 3 categories: no renal occlusion, WIT <30 minutes and WIT >30 minutes. This revealed that based on postoperative serum creatinine, WIT of up to 55 minutes does not impact on long term renal function after partial nephrectomy. It concluded that efforts to limit WIT are important but must not compromise cancer control, hemostasis and collecting system reconstruction. The study’s population is representative of the majority of patients who present with a solitary renal mass and have bilaterally functioning kidneys, hence it gives the average urologist the most practical and attainable advice with regards to WIT and its impact on postop renal function. Its results however is debunked by Funahashi et al’s study that clearly defined 25 minutes of WIT as the cut off to ensure maximum preservation of renal function.
Papalia et al. [7] presents their experience with the use of anesthesia to avoid hilar clamping. Gill’s group initially used this technique as a pilot study to study global hypotension to achieve “zero ischemia” during laparoscopic partial nephrectomy. This technique was criticizes as being potentially risky for patients and not very widely applicable and was abandoned in favor of achieving zero global ischemia via microvascular dissection, isolation and clamping of the quarternary arterial supply of the tumor region. In Papalia’s study, 60 patients underwent laparoscopic and robotic partial nephrectomy without hilar clamping through the use of controlled hypotensive anesthesia. The median mean arterial pressure was maintained at 65 mm Hg during the excision of the tumor and subsequent repair of the defect. Their average hypotensive time was 14 minutes. All cases had negative surgical margins. 6.6% however required postoperative transfusion. 3 patients developed complications ranging from port site bleeding, hemorrhage and hematoma. The median range in estimated glomerular filtration rate was 11.6 ml/minute/1.73 meter squared. The authors consider their results encouraging. The concept of global hypotension is brilliant however, and certainly pioneering as it establishes certain boundaries. Credit should be given for the innovativeness and attempt. Some, however, may feel that these complications were an unnecessary trade off just to avoid clamping. Nevertheless, it still presents a novel way of limiting the use of mechanically induced ischemia.
Porpiglia et al. [9] study prospectively followed up 54 patients who underwent partial nephrectomy. It made use of renal scintigraphy (MAG-3) to access renal function after surgery. The study revealed that split renal function and effective renal plasma flow decreased by 4% and 29 ml/min/meter squared after 3 months but remained stable onward for up to the 4th year duration of the study. Using a regression model to interpret their statistical data, they found out that at 3 months WIT and age had a significant impact on postoperative renal function, however at 48 months into the study, only WIT had a strong statistical correlation with renal function. This study however did not describe the WIT cut off to minimize ischemic injury. It also did not take into account the percentage of the renal parenchyma preserved.
Bollens et al. [10] study investigated the use of “on demand” clamping. In a series of 39 patients who underwent laparoscopic partial nephrectomy, they only performed hilar clamping according to the authors as needed. 31 patients required hilar clamping but the authors also employed immediate release as soon as the defect was repaired. Their median WIT was 9 minutes. Transfusions were required in 20% of the patients and 2 cases were converted to open surgery. Although, we commend the authors for their innovative technique, we feel that the expense of incurring unnecessary perioperative complications makes justification of this method difficult.
Funashashi et al. [11] study made use of MAG-3 to evaluate renal function in 32 patients who underwent partial nephrectomy with conventional hilar clamping. The test was done at 1 week and 6 months after surgery. Their results revealed that WIT of more than or equal to 25 minutes caused irreversible diffuse parenchymal damage and that patients exposed to WIT <25 minutes demonstrated recovery of renal function. This study supports Campbell's [18] argument that when one does not take into account the percent of renal parenchyma preserved then WIT would be given the surrogate role of being the predictor of postoperative renal function. It also did not make use of any anatomic classification systems to describe the tumor characteristics. These omissions deprived the study of the vital contribution that these factors play in determining ultimate renal function. This study however, finds utility by clearly defining the safe WIT.
Holzer et al. [12] study documented the use of Digital Light Processing (DLP) hyperspectral imaging as a real time noninvasive method to assess renal oxyhemoglobin saturation intraoperatively throughout the kidney. It demonstrated that within 10 minutes of hilar occlusion oxyhemoglobin levels reached its nadir. The experiment also illustrated that oxyhemoglobin returned to baseline after a median of 5.8 minutes after unclamping. This novel device and new information could facilitate the development of future surgical interventions that can titrate ischemia so as to minimize injury in real time.
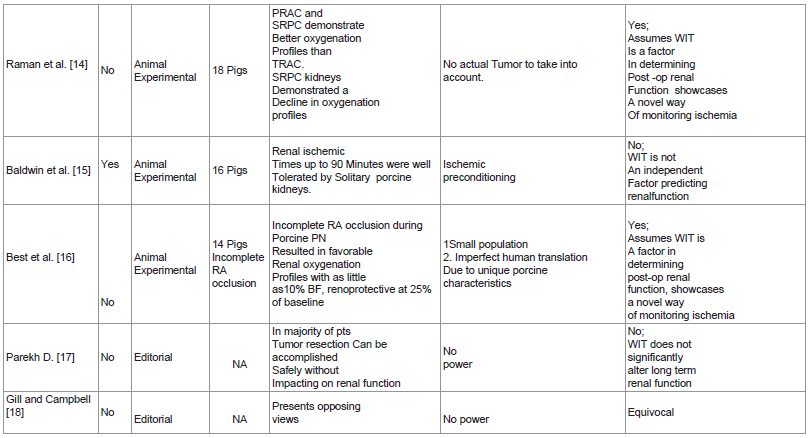
Raman et al. [14] study described the use of a Licox probe to monitor renal partial oxygen pressure while doing partial nephrectomy in porcine models relegated to 3 treatment arms: total renal artery clamping, partial renal artery clamping and selective renal parenchymal clamping. Their results revealed favorable intraoperative oxygenation profles in the partial renal artery clamping group as well as the selective renal parenchymal clamping group. Post operative creatinine levels also revealed a significant increase in the selective renal parenchymal clamping group. This study also presents another innovative way for real time assessment and titration of kidney perfusion during partial nephrectomy.
Baldwin et al. [15] study demonstrated the effect of pneumoperitoneum in laparoscopic surgery and its impact on WIT in solitary kidney porcine models. They divided 16 pigs into 4 treatment arms differing in their WIT: 0, 30, 60 and 90 minutes. Their study revealed transient serum creatinine increase in the 60 and 90 minute groups at day 2 and 4 postop. Normal creatinine levels however were observed in all the treatment arms by day 7 postop. They also concluded that WIT of up to 90 minutes is well tolerated by porcine kidneys. They attributed this phenomenon to the potential protective effect of relative ischemic preconditioning provided by the pneumoperitoneum. This study though well designed has the serious limitation of having results which are applicable only in the animal model on which it was conducted.
Best et al. [16] study documented the utility of Hyperspectral Imaging (HSI) in monitoring tissue oxygenation in solitary kidney porcine models which underwent open partial nephrectomy with warm ischemia. Using an adjustable renal clamp they divided their population into 3 treatment arms based on the residual renal artery flow after clamping: 0%, 10% and 25%. The study revealed that incomplete renal artery occlusion resulted in favorable oxygenation profiles. It also demonstrated the renoprotective effect of maintaining 25% renal arterial flow. The novel method of using HSI in combination with an adjustable renal artery clamp to titrate and maintain renal perfusion during partial nephrectomy puts a new dimension into the issue of hilar clamping and ischemia in nephron sparing surgery. This technique presents an alternative way of doing hillar clamping. Controlled or titrated hilar clamping combined with the knowledge of a definitive WIT cut off of 25 minutes would hopefully usher in the age of maximum quantity and quality renal preservation.
Conclusion
This review of articles of interest discussing the issue of the impact of WIT on renal function brings into focus the different technical developments and innovations currently revolutionizing the way we conduct our surgeries. We live in an exciting time where technological advancement complements our efforts to constantly find better ways to cure our patients. The debate presented and discussed in this review showcases the passion of personalities involved in setting high standards surgical skills development. It also demonstrates the strict requirement of maintaining the acceptable risks and complications that the current standard of care provide. It is our fervent hope that these dynamic exchange of ideas would ultimately benefit our patients by raising the bar of healthcare.
References
- Sun M, Bianchi M, Hansen J, Trinh QD, Abdollah F, et al. (2012) Chronic kidney disease after nephrectomy in patients with small renal masses: a retrospective observational analysis. Eur Urol .
- Weight C, Lieser G, Larson B, Gao T, Lane BR, et al. (2010) Partial nephrectomy is associated with improved overall survival compared to radical nephrectomy in patients with unanticipated benign renal tumours. Eur Urol 58: 293-298.
- Touijer K, Jacqmin D, Kavoussi LR, Montorsi F, Patard JJ, et al. (2010) The expanding role of partial nephrectomy: a critical analysis of indications, results, and complications. Eur Urol 57: 214-222.
- Lane BR, Russo P, Uzzo RG, Hernandez AV, Boorjian SA, et al. (2011) Comparison of cold and warm ischemia during partial nephrectomy in 660 solitary kidneys reveals predominant role of nonmodifiable factors in determining ultimate renal function. J Urol 185: 421-427.
- Spana G, Haber GP, Dulabon LM, Petros F, Rogers CG, et al. (2011) Complications after robotic partial nephrectomy at centers of excellence: multiinstitutional analysis of 450 cases. J Urol 186: 417-421.
- Bhayani SB, Rha KH, Pinto PA, Ong AM, Allaf ME, et al. (2004) Laparoscopic partial nephrectomy: effect of warm ischemia on serum creatinine. J Urol 172: 1264-1266.
- Papalia R, Simone G, Ferriero M, Costantini M, Guaglianone S, et al. (2012) Laparoscopic and robotic partial nephrectomy with controlled hypotensive anesthesia to avoid hilar clamping: feasibility, safety and perioperative functional outcomes. J Urol 187: 1190-1194.
- Gill IS, Patil MB, Abreu AL, Ng C, Cai J, et al. (2012) Zero ischemia anatomical partial nephrectomy: a novel approach. J Urol 187: 807-814.
- Porpiglia F, Fiori C, Bertolo R, Morra I, Russo R, et al. (2012) Long-term functional evaluation of the treated kidney in a prospective series of patients who underwent laparoscopic partial nephrectomy for small renal tumors. Eur Urol 62: 130-135.
- Bollens R, Rosenblatt A, Espinoza BP, De Groote A, Quackels T, et al. (2007) Laparoscopic partial nephrectomy with “on-demand” clamping reduces warm ischemia time. Eur Urol 52: 804-809.
- Funahashi Y, Hattori R, Yamamoto T, Sassa N, Fujita T, et al. (2012) Effect of warm ischemia on renal function during partial nephrectomy: assessment with new 99mtc- mercaptoacetyltriglycine scintigraphy parameter. Urology 79: 160–164.
- Holzer MS, Best SL, Jackson N, Thapa A, Raj GV, et al. (2011) Assessment of renal oxygenation during partial nephrectomy using hyperspectral imaging. J Urol 186: 400-404.
- Gill IS, Eisenberg MS, Aron M, Berger A, Ukimura O, et al. (2011) “Zero ischemia” partial nephrectomy: novel laparoscopic and robotic technique. Eur Urol 59: 128-134.
- Raman JD, Bensalah K, Bagrodia A, Tracy CR, Kabbani W, et al. (2009) Comparison of tissue oxygenation profiles using 3 different methods of vascular control during porcine partial nephrectomy. Urology 74: 926-931.
- Baldwin DD, Maynes LJ, Berger KA, Desai PJ, Zuppan CW, et al. (2004) Laparoscopic warm renal ischemia in the solitary porcine kidney model. Urology 64: 592-597.
- Best SL, Thapa A, Holzer MJ, Jackson N, Mir SA, et al. (2011) Minimal arterial in-flow protects renal oxygenation and function during porcine partial nephrectomy: confirmation by hyperspectral imaging. Urology 78: 961-966.
- Parekh DJ (2012) Warming up to ischemia. J Urol 187: 785-786.
- Aron M, Gill IS, Campbell SC (2012) A nonischemic approach to partial nephrectomy is optimal. Yes. J Urol 187: 387-388.
- Gill IS, Kamoi K, Aron M, Desai MM (2010) 800 Laparoscopic partial nephrectomies: a single surgeon series. J Urol 183: 34-41.
- Lane BR, Gill IS, Fergany AF, Larson BT, Campbell SC (2011) Limited warm ischemia during elective partial nephrectomy has only a marginal impact on renal functional outcomes. J Urol 185: 1598-1603.
- Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, et al. (2010) Every minute counts when the renal hilum is clamped during partial nephrectomy. Eur Urol 58: 340-345.
*Corresponding author: Arturo P. Castro Jr, Division of Endourology, Robotics, Laparoscopy and Minimally Invasive Surgery, USA
Citation: Castro Jr AP, Gorbatiy V, Leveillee RJ (2012) Recent Advances in Nephron Sparing Surgery: A Review of Articles of Interest Discussing the Impact of Ischemia in the Preservation of Renal Function. Med Surg Urol S4:001. doi:10.4172/2168-9857.S4-001


 (6 votes, average: 4.33 out of 5)
(6 votes, average: 4.33 out of 5)